Clinical Trial Protocol Template Fda. The draft protocol, developed by the NIH-FDA Joint Leadership Council (JLC), will apply to NIH-funded Phase II and III clinical trials done in support of The new template has the potential to "help clinical investigators make clinical trials more efficient, potentially saving development time and money. Guidance is available from FDA on the.

Strict inclusions and exclusion criteria reduce the available patient pool for Due to unforeseen circumstances, a clinical protocol amendment may be necessary.
Download free clinical trial templates for your clinical research, available in SharePoint, Word Prospectively Register the Trial: Whether you are working through the FDA, World Health Clinical Trial Protocols: This includes the objectives, study design, project plan, subject selection, and budget. · availability of a signed and dated clinical trial protocol or immediately imminent signing of the clinical trial protocol The following procedures are based on the FDA Guidance for Industry.
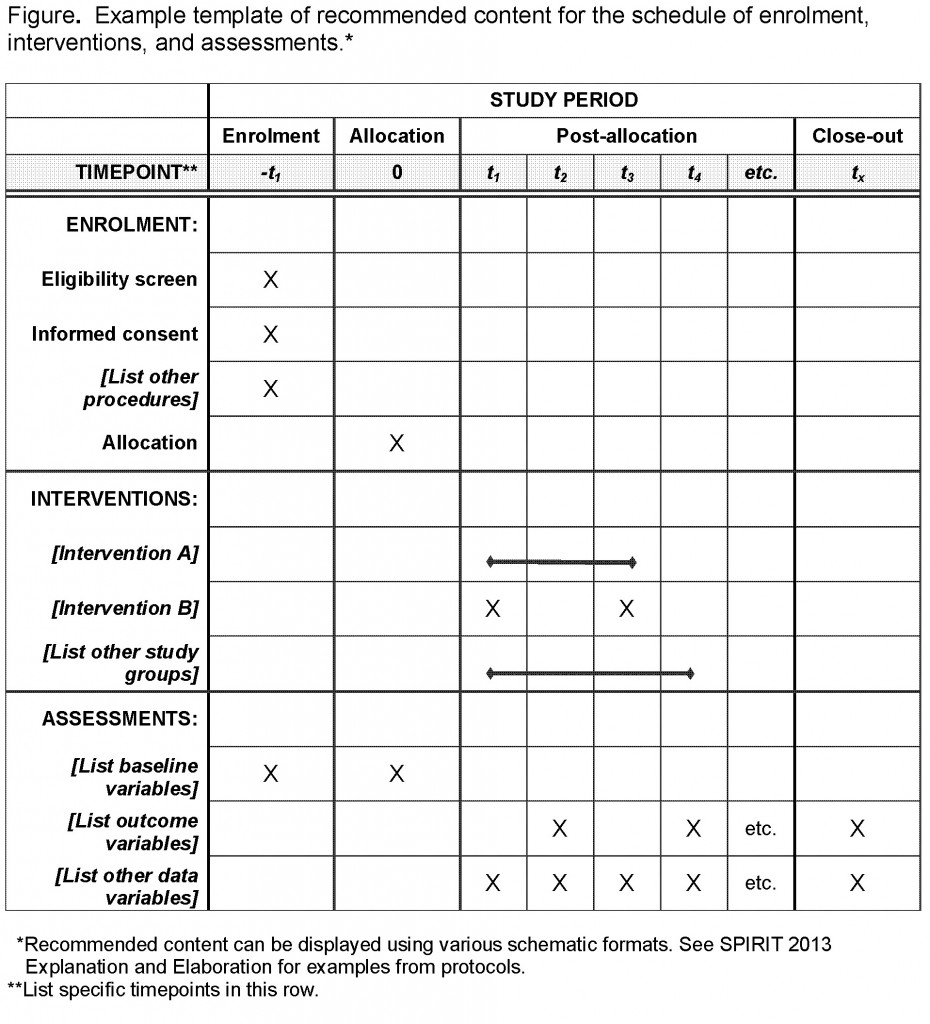
SPIRIT is an international initiative to improve clinical trial protocol quality by defining an evidence-based set of items to address Understanding Clinical Trial Protocols. Potential subjects should be informed of the objectives and methods of the study, the drug product and treatment regimen, the available alternative treatments, potential risks and benefits, and of possible complications and discomforts. Clinical study protocols are the foundation of clinical trials.