Clinical Trial Protocol Template Eu. NCI Informed Consent Template for Adult Cancer Trials using the ETCTN Biorepository and/or National Clinical Laboratory Network (NCLN) (MS Word) — ETCTN investigators utilizing the ETCTN Biorepository and/or NCLN should use this version of the NCI. Each Investigator participating in the clinical trial and the associated clinical study staff will receive training on the clinical protocol.

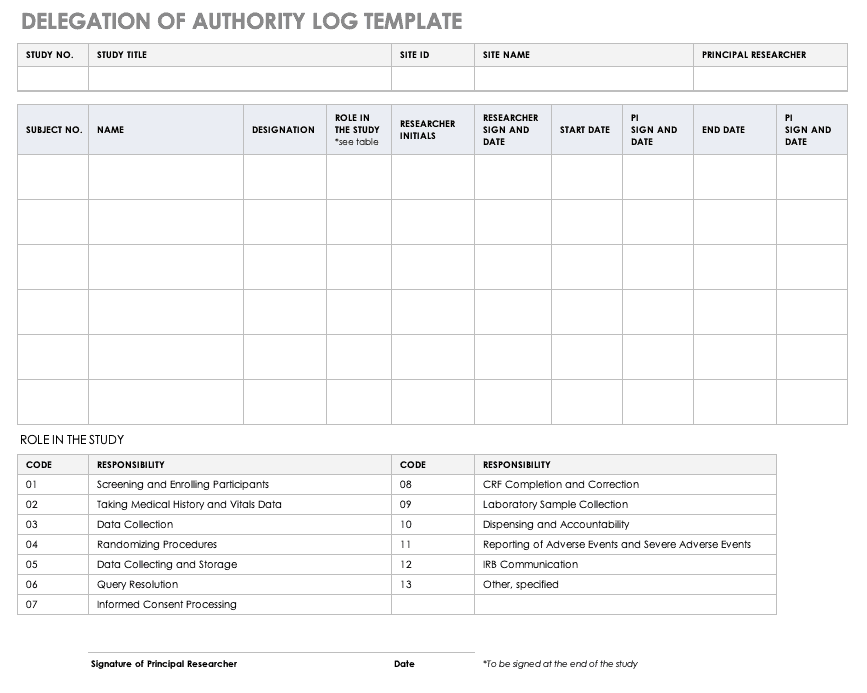
The Clinical Trials Unit at Kilimanjaro Christian Medical Center has prepared numerous Standard Operating Procedures (SOPs) for clinical trials that meet NIH requirements.
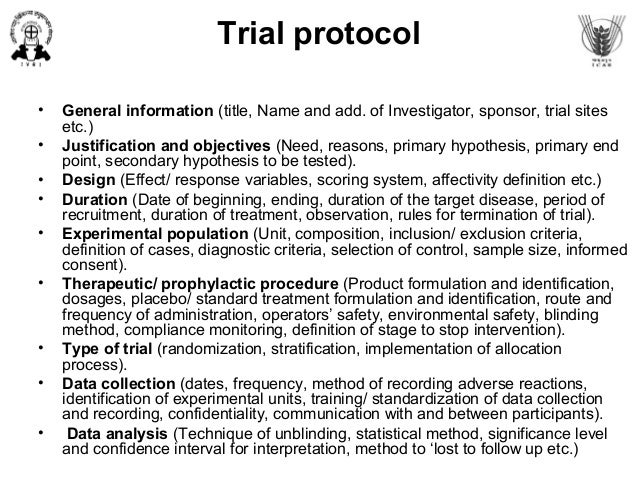
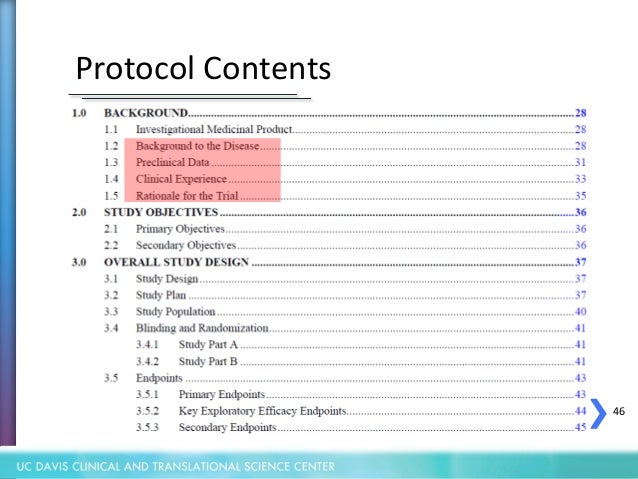
To meet Good Clinical Practice Guidelines the Protocol should contain, but not be restricted to, the information contained within this template.
The clinical trial data collection at Pharma Intelligence includes clinical trial data sets, clinical research information German, Danish Regulators Explain Dos And Don'ts Of Master Protocols. The clinical trial template has site lists of libraries for clinical trial protocols, protocol documents, announcements, calendars, issues, tasks, and document discussions. These can be further customized with different versions of SharePoint.