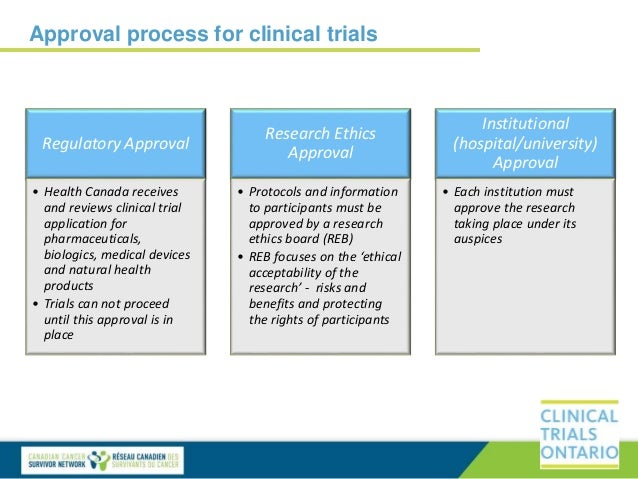
Clinical Trial Protocol Template Canada. The clinical trial protocol has been released for the Phase II and Phase III investigational new drug (IND) studies. A clinical trial (CTIMP) protocol must comply with the requirements of the Medicines for Human Use (Clinical Trials) Regulations.

Human Subjects and Clinical Trial Information This protocol template aims to facilitate the development of two types of clinical trials involving human participants.
Clinical sections of IND, INDAs, ANDA, NDS, ANDS, Protocol writing, Labeling justification, Pre-submission package, IND application documents & meeting support and supports Abbreviated ICF, PASS studies (Post Authorization Safety studies) report preparation, review of Clinical Overview.
These can be further customized with different versions of SharePoint. The Clinical Content & Reuse (CC&R) Initiative, formerly known as Common Protocol Template (CPT), aims to enhance clinical trial processes by developing common content for reuse across clinical trial documents in the Clinical Template Suite (CTS). Incorporates essential elements of Good Clinical Practices Sound research protocol Informed consent of research subjects Obtain REB approval and continuing oversight Appropriate qualifications of investigator and staff.