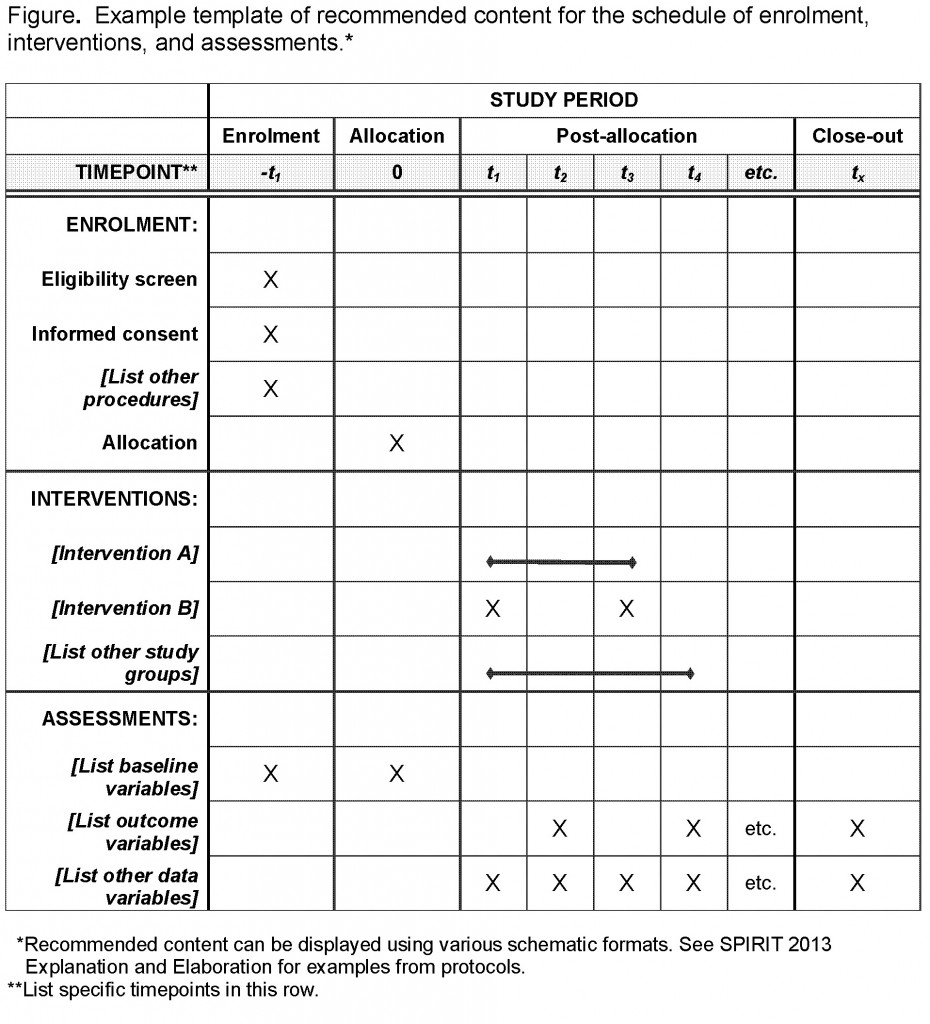
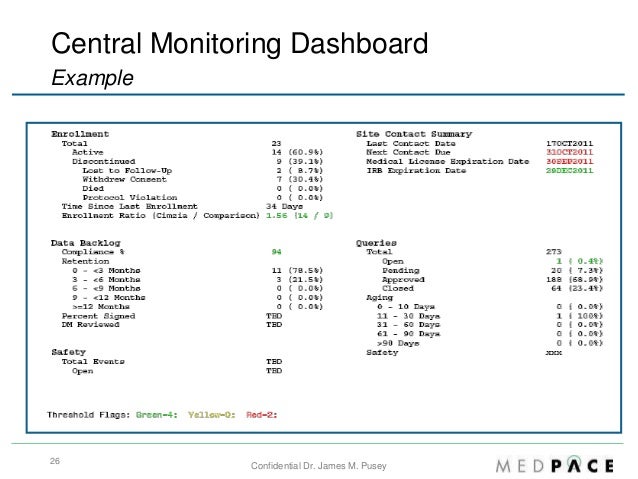
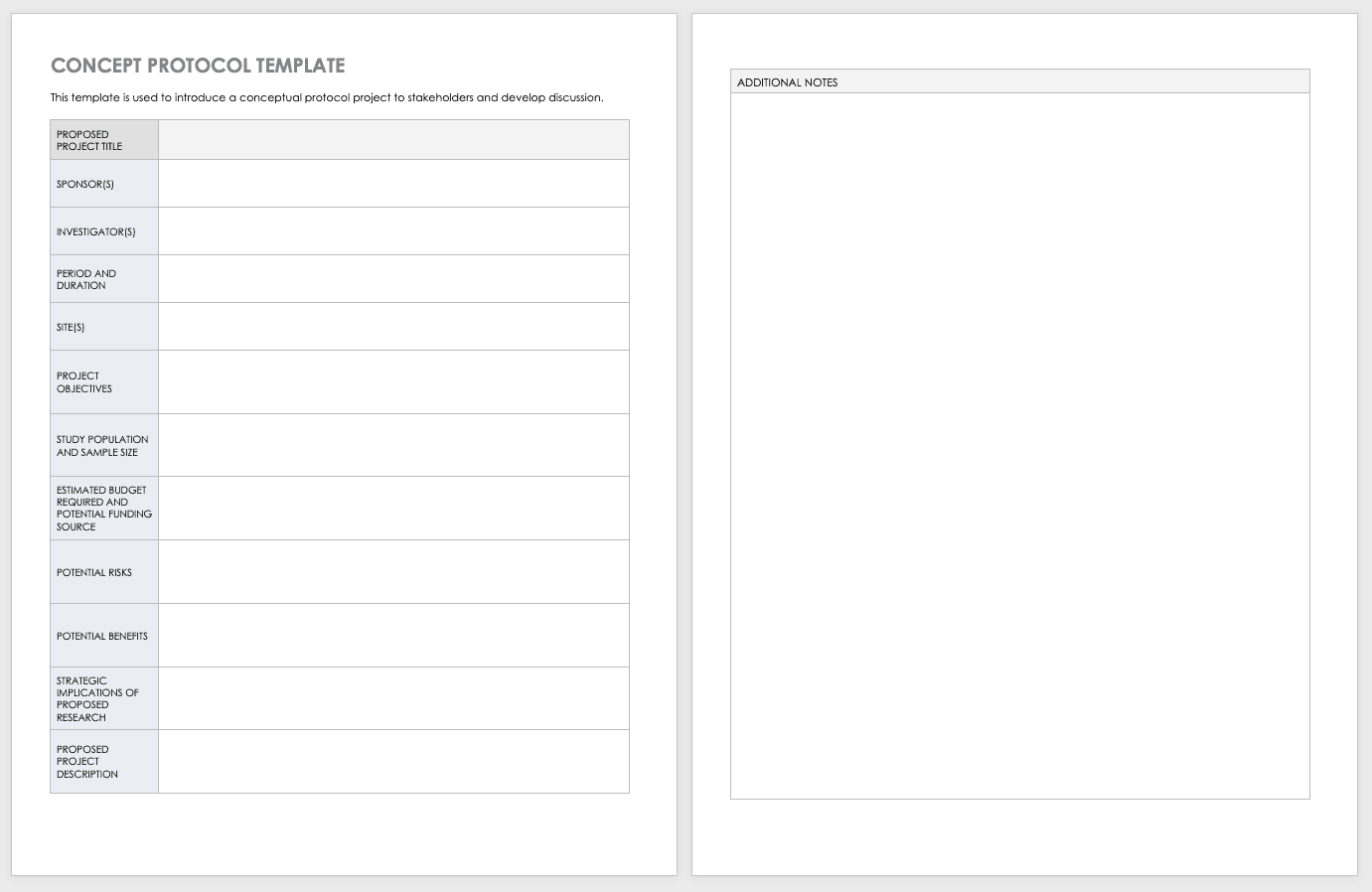
Clinical Trial Protocol Template. Each Investigator participating in the clinical trial and the associated clinical study staff will receive training on the clinical protocol. To download this template, you will need access to SharePoint.

Background All randomized clinical trials (RCTs) require a protocol; however, numerous studies have highlighted protocol deficiencies.
Each Investigator participating in the clinical trial and the associated clinical study staff will receive training on the clinical protocol.
These clinical protocol templates can be accessed via the secure web-based e-Protocol Writing Tool and as Word templates. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about. NCI Informed Consent Template for Adult Cancer Trials using the ETCTN Biorepository and/or National Clinical Laboratory Network (NCLN) (MS Word) — ETCTN investigators utilizing the ETCTN Biorepository and/or NCLN should.