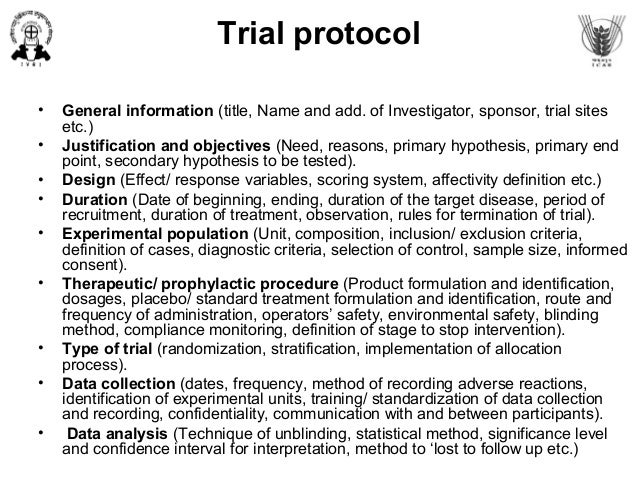
Clinical Study Protocol Template Ema. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. The clinical study report (CSR) is It is currently noted that the suspension is temporary and EMA will announce when activities are Essential documents list (pre-writing): • Study protocol and protocol amendments • Clinical study report template (and any.
An Insider's Guide to Clinical Study Reports.
The online protocol template called SEPTRE (SPIRIT Electronic Protocol Tool & Resource) is a web-based option to create, manage, and register Trials requires the submission of a populated SPIRIT checklist and figure for all clinical trial study protocols, except those submitted using the optional.
We are currently undertaking a program of research aimed at better understanding the role of systematic. By publishing your protocol in BMC Public Health, it becomes a fully citable. Using a Protocol Template for Case Study Planning.